🚚 Global express, reliable and continuous availability of medical supplies and PPE for healthcare systems, hospitals and more.
Menu
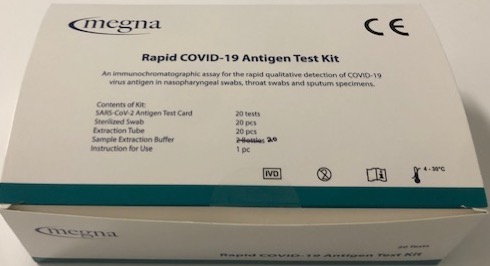
Rapid COVID-19 Antigen Test Kit
Key Features
- FDA Sample: NP swab
- Sensitivity 97% – combined
- Sensitivity 100% – combined
- Made in USA
- Rapid results: 10 mins
- No additional equipment needed
- Result: positive indicates current infection
- CE Mark
- Authorized by the FDA under a EUA for use by authorized laboratories.
- This test has not been FDA cleared or approved
- This test is only authorized for the duration of the declaration that
circumstances exist justifying the authorization of emergency use
of in vitro diagnostic tests for detection and/or diagnosis of COVID-19
under Section 564(b)(1) of the Act,21 U.S.C. § 360bbb-3(b)(1), unless
the authorization is terminated or revoked sooner.
What’s included
- Test cards (20)
- Extract solution (20)
- Extract tubes (20)
- Nasal swabs (20)
- Tube rack for specimens
- Instructions for use
Packaging & Order Information
- 20 test kits per box; room temperature storage
Category: COVID-19 Test Kits