🚚 Global express, reliable and continuous availability of medical supplies and PPE for healthcare systems, hospitals and more.
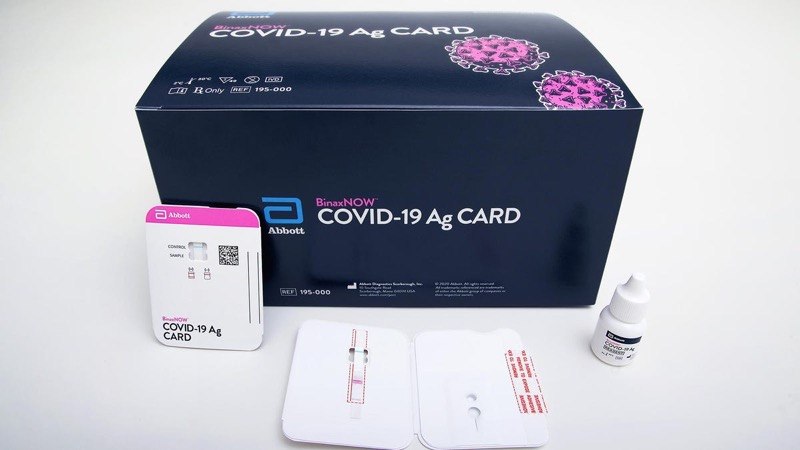
Abbott Laboratories Rapid COVID-19 Test
BinaxNOW™ Professional Use Antigen Detection COVID-19 Ag Nasal Swab Sample 40/Pk
BinaxNOW COVID-19 Ag Card is only for use under the Food and Drug Administration’s Emergency Use Authorization: https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations
Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high or waived complexity tests; this test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation
The BinaxNOW COVID-19 Ag Card is a lateral flow immunoassay for the qualitative detection of the nucleocapsid protein antigen to SARS-CoV-2 directly from anterior nasal (nares) swab specimens collected from individuals who are suspected of COVID-19 by their healthcare provider within seven days of the onset of symptoms
Laboratories within the United States and its territories are required to report all results to the appropriate public health authorities
Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status
Positive results do not rule out bacterial infection or co-infection with other viruses
Negative results should be treated as presumptive and confirmation with a molecular assay, if necessary, for patient management, may be performed
Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions
The BinaxNOW COVID-19 Ag Card is intended for use by medical professionals or trained operators who are proficient in performing rapid lateral flow tests
The BinaxNOW COVID-19 Ag Card does not differentiate between SARSCoV and SARS-CoV-2
- Sensitivity (PPA) 84.6% (entire population)
- Sensitivity (PPA) 95.6% (those with PCR cycle threshold [Ct] < 33)
- Specificity (NPA) 98.5%
Onboard extraction allows the swab to be directly inserted into the test card
Visually read results in 15 minutes – no instrument required
Model: 195000